Rainbow in the Dark: Powerful proof of 9/11 Nukes
New research falsifies the nanothermite and DEW alternatives
while confirming the mini-nuke hypothesis
http://www.veteranstoday.com/2015/02/06/rainbow-in-the-dark-powerful-proof-of-911-nukes/


(h1) the nanothermite hypothesis, supported by A&E911, Steve Jones and Christopher Bollyn;
(h2) the mini-nuke hypothesis, supported by Scholars for 9/11 Truth and others here at VT;
(h3) the Directed Energy Weapon (DEW) hypothesis, supported by Judy Wood and her associates.
A&E911 has received enormous support from the public, which has allowed its head, Richard Gage, to travel around the country and lead a comfortable life-style. His critics, however, are skeptical that A&E911 is on the up-and-up because its commitment to nanothernmite has been established to be inadequate as an explanation for the destruction of the Twin Towers, not least of all because in (its extant versions) nanothermite has an explosive force 1/13 as powerful as TNT. While A&E911 apologists maintain the organization does not deny other explosives may have been involved, it has made no effort to ascertain what those “other explosives” could possibly have been, which is inexcusable.
That is rather astonishing, all things considered, because (h1) has been based (virtually exclusively) upon dust samples that were gathered from an apartment in close proximity to “Ground Zero”, where the US Geological Survey has done overwhelmingly more through, detailed and extensive studies of dust samples take from a wide range of locations around Manhattan and subjected them to spectrographic analysis, which has revealed many elements whose presence in the quantities and correlations only appear to be explicable if 9/11 was nuclear event as (h2) asserts:
Donald Fox has done a brilliant job of exposing the role of Judy Wood as a “gatekeeper extraordinaire”, where the A&E911 (h1) group cannot be far behind. Unfortunately, a gullible public swoons over an organization composed of a thousand or more architects and engineers, even though they will not address WHO was responsible and WHY 9/11 took place and, like Judy Wood and her (h3) group, they do not even provide an adequate explanation for HOW it was done. Here Don Fox advances a new line of argument confirming the use of nukes on 9/11 in support of (h2). This article has a more scientific and technical character than others, where we conclude with a glossary of terms related to the design, construction and detonation of nuclear weapons, the popularization of which Gordon Duff has pioneered, which, alas, must become part of the working knowledge of informed citizens today.

The evidence collected at Ground Zero tells us 9/11 was a nuclear event. The Department of Energy water samples contained tritium which is a rare hydrogen isotope and a telltale sign of a thermonuclear explosion. The USGS dust samples contained barium, strontium, yttrium and cesium amongst other fission products. The earth and water tell us what happened on 9/11: the WTC was nuked.
Most 9/11 researchers (and the general public) lack a basic understanding of nuclear physics. This lack of physics knowledge has allowed people like Steve Jones and Judy Wood to obfuscate the true method of destruction of the WTC buildings with bogus claims of explosive nanothermite and amorphous DEWs. Once one gains an understanding of concepts such as nuclear fission and alpha and beta decay, the destruction of the Twin Towers and the aftermath makes perfect sense.
 In order to understand what happened at Ground Zero one must first
have a grasp on some basic nuclear physics concepts. Fortunately there
is an abundance of nuclear physics knowledge readily available on the
internet. From Universe Today.com:
In order to understand what happened at Ground Zero one must first
have a grasp on some basic nuclear physics concepts. Fortunately there
is an abundance of nuclear physics knowledge readily available on the
internet. From Universe Today.com:
There are three parts of an atom: protons, neutron, and electrons. Protons have a positive charge, electrons have a negative charge, and neutrons possess no net charge.
Electrons are the smallest parts of the atom. They are the most numerous of the three. It has no known components or substructure, so it is an elementary particle. Its mass is 1/1836 of a proton. It is also considered to be a fermion. It has an antiparticle called the positron. The positron is identical to the electron except that it carries opposite charge. When an electron collides with a positron, both particles will either scatter or be destroyed producing gamma ray photons. Electrons can collide with other particles and be diffracted like light. Two electrons cannot occupy the same quantum state based on the Pauli Exclusion Principle.
The proton is the part of an atom that helps to form the nucleus and has a positive charge. Protons must have an equal number of neutrons except in the hydrogen atom where a single proton exists on its own. A proton is composed of 2 up quarks and one down quark. They are considered to be fermions and baryons. They are held together by the strong nuclear force. The number of protons in the nucleus of an atom determines the atomic number.
A neutron is the part of an atom that holds no charge. Neutrons and protons occur in equal numbers in stable atoms except in hydrogen. Protons and neutrons are often referred to together as nucleons. If there are more neutrons than protons, then the atom is considered an isotope. If a neutron becomes free of its proton, then it becomes unstable, undergoes beta decay, and will disintegrate in an average of 15 minutes. The neutron is also important in nuclear chain reactions both natural and artificial.
Extensive studies by Charles Boldwyn have demonstrated that the Twin Towers could not have collapsed on 9/11.
From Chemistry Explained.com: The nucleus is composed of protons (charge = +1; mass = 1.007 atomic mass units ([μ]) and neutrons. The number of protons in the nucleus is called the atomic number (Z) and defines which chemical element the nucleus represents. The number of neutrons in the nucleus is called the neutron number (N), whereas the total number of neutrons and protons in the nucleus is referred to as the mass number (A), where A = N + Z. The neutrons and protons are referred to collectively as nucleons. A nucleus with a given N and Z is referred to as a nuclide. Nuclides with the same atomic number are isotopes, such as 12 C and 14 C, whereas nuclides with the same N, such as 14 C and 16 O, are called isotones. Nuclei such as 14 N and 14 C, which have the same mass number, are isobars. Nuclides are designated by a shorthand notation in which one writes, that is, for a nucleus with 6 protons and 8 neutrons, one writes, or just 14 C. The size of a nucleus is approximately 1 to 10 × 10 -15m, with the nuclear radius being represented more precisely as 1.2 × A 1/3 × 10 -15m. We can roughly approximate the nucleus as a sphere and thus we can calculate its density where 1.66 × 10-27 kg is the mass of the nucleon. Thus the nuclear density is about 200,000 tons per cubic millimeter and is independent of A. Imagine a cube that is 1 mm on a side. If filled with nuclear matter, it would have a mass of about 200,000 tons. This calculation demonstrates the enormous matter/energy density of nuclei and gives some idea as to why nuclear phenomena lead to large energy releases.
Of the 6,000 species of nuclei that can exist in the universe, about 2,700 are known, but only 270 of these are stable. The rest are radioactive, that is, they spontaneously decay. The driving force behind all radioactive decay is the ability to produce products of greater stability than one had initially. In other words, radioactive decay releases energy and because of the high energy density of nuclei, that energy release is substantial. Qualitatively we describe radioactive decay as occurring in three general ways: α -, β -, and γ -decay. Alpha-decay occurs in the heavy elements, and consists of the emission of a 4 He nucleus. Beta-decay occurs in nuclei whose N/Z ratio is different from that of a stable nucleus and consists of a transformation of neutrons into protons or vice versa to make the nucleus more stable. Gamma-decay occurs when excited nuclei get rid of some or all of their excitation energy via the emission of electromagnetic radiation, or via the radiationless transfer of energy to orbital electrons.
 probable
fragment masses are around mass 95 and 137. Most of these fission
fragments are highly unstable (radioactive), and some of them such as cesium-137 and strontium-90 are extremely dangerous when released to the environment.
probable
fragment masses are around mass 95 and 137. Most of these fission
fragments are highly unstable (radioactive), and some of them such as cesium-137 and strontium-90 are extremely dangerous when released to the environment.
Cesium-137 and strontium-90 are the most dangerous radioisotopes to the environment in terms of their long-term effects. Their intermediate half-lives of about 30 years suggests that they are not only highly radioactive but that they have a long enough half-life to be around for hundreds of years. Iodine-131 may give a higher initial dose, but its short half-life of 8 days ensures that it will soon be gone. Besides its persistence and high activity, cesium-137 has the further insidious property of being mistaken for potassium by living organisms and taken up as part of the fluid electrolytes. This means that it is passed on up the food chain and re-concentrated from the environment by that process.
Cesium-137 decay has a half-life of 30.07 years and proceeds by both beta decay and gamma emission from an intermediate state. Both the electron and gamma emissions are highly ionizing radiation. The gamma radiation is very penetrating, and the beta radiation, though very short range, is very dangerous when ingested because it deposits all that energy in a very short distance in tissue.
Cesium’s danger as an environmental hazard, damaging when ingested, is made worse by its mimicking of potassium’s chemical properties. This ensures that cesium as a contaminant will be ingested, because potassium is needed by all living things.
Strontium-90 and cesium-137 are the radioisotopes which should be most closely guarded against release into the environment. They both have intermediate half-lives of around 30 years, which is the worst range for half-lives of radioactive contaminants. It ensures that they are not only highly radioactive but also have a long enough half-life to be around for hundreds of years. Strontium-90 mimics the properties of calcium and is taken up by living organisms and made a part of their electrolytes as well as deposited in bones. As a part of the bones, it is not subsequently excreted like cesium-137 would be. It has the potential for causing cancer or damaging the rapidly reproducing bone marrow cells.
Strontium-90 is not quite as likely as cesium-137 to be released as a part of a nuclear reactor accident because it is much less volatile, but is probably the most dangerous component of the radioactive fallout from a nuclear weapon.
Strontium-90 undergoes beta decay, emitting electrons with energy 0.546 MeV with a half-life of 28.8 years. The decay product is yttrium-90.
Beta particles are electrons or positrons (electrons with positive electric charge, or anti-electrons). Beta decay occurs when, in a nucleus with too many protons or too many neutrons, one of the protons or neutrons is transformed into the other. In beta minus decay, a neutron decays into a proton, an electron, and an anti-neutrino: n Æ p + e – +. In beta plus decay, a proton decays into a neutron, a positron, and a neutrino: p Æ n + e+ +n. Both reactions occur because in different regions of the Chart of the Nuclides, one or the other will move the product closer to the region of stability.

These particular reactions take place because conservation laws are obeyed. Electric charge conservation requires that if an electrically neutral neutron becomes a positively charged proton, an electrically negative particle (in this case, an electron) must also be produced. Similarly, conservation of lepton number requires that if a neutron (lepton number = 0) decays into a proton (lepton number = 0) and an electron (lepton number = 1), a particle with a lepton number of -1 (in this case an antineutrino) must also be produced. The leptons emitted in beta decay did not exist in the nucleus before the decay–they are created at the instant of the decay.
To the best of our knowledge, an isolated proton, a hydrogen nucleus with or without an electron, does not decay. However within a nucleus, the beta decay process can change a proton to a neutron. An isolated neutron is unstable and will decay with a half-life of 10.5 minutes. A neutron in a nucleus will decay if a more stable nucleus results; the half-life of the decay depends on the isotope. If it leads to a more stable nucleus, a proton in a nucleus may capture an electron from the atom (electron capture), and change into a neutron and a neutrino.
Proton decay, neutron decay, and electron capture are three ways in which protons can be changed into neutrons or vice-versa; in each decay there is a change in the atomic number, so that the parent and daughter atoms are different elements. In all three processes, the number A of nucleons remains the same, while both proton number, Z, and neutron number, N, increase or decrease by 1.
In beta decay the change in binding energy appears as the mass energy and kinetic energy of the beta particle, the energy of the neutrino, and the kinetic energy of the recoiling daughter nucleus. The energy of an emitted beta particle from a particular decay can take on a range of values because the energy can be shared in many ways among the three particles while still obeying energy and momentum conservation.
When a charged particle does move through a medium at a speed higher than the speed of light in that medium, a faint radiation is produced by the medium. In water, for example, the charged particle excites the water molecules, which then return to their normal state by emitting photons of blue light. Because the particle is moving faster than the speed of light in water, it can trigger a cascade of photons that are in phase with each other and can interfere constructively to form a visible blue glow.

Nuclear Waste Encapsulation and Storage Facility, Cherenkov Radiation, Hanford Site, Southeastern Washington State
Cesium-137 and Strontium-90 Capsules
In the early 1970s operators at the Hanford, Washington, site removed a large fraction of the Cs-137 and Sr-90 from the site’s high-level tank waste in order to reduce the requirements for cooling the tanks. The cesium and strontium were concentrated and sealed in stainless steel (SS) capsules for potential uses, for example, thermoelectric generators and sterilizers. The expected applications for the Hanford capsules did not materialize and ceased entirely in 1988 after one capsule being used in the commercial sector was found to be leaking (USNRC, 1989).

In the capsules, cesium is in the form of cesium chloride (CsCl) and strontium as strontium fluoride (SrF2). The chemical composition has been described as being relatively uniform (NRC, 1997b). Each cesium capsule contains on average approximately 35,000 Ci of Cs-137 plus an unspecified amount of Cs-135 estimated to be 0.7 Ci and produces approximately 190 W of heat. Each strontium capsule contains approximately 33,000 Ci of Sr-90 and produces approximately 260 W of heat.
The blue glow from the cesium-137 and strontium-90 capsules is due to Cherenkov radiation.
Cesium-137 Glows Blue
Majia Nadesan has a blog post that discusses the Goiânia accident where a scavenger punctured a radiotherapy device containing cesium-137 with a screwdriver. A deep blue light came out of the hole.
Cesium and Strontium in the USGS Dust Samples
Both cesium and strontium were found in abundance in the USGS dust samples. Both cesium-137 and strontium-90 undergo β− decay. Cesium-137 decays into barium-137m and strontium-90 decays into yttrium-90. Barium and yttrium were also found in abundance in the USGS dust samples. There can be little doubt that the ominous blue glow in the above picture of Ground Zero is from Cherenkov radiation.

Beautiful, but misleading. The light memorial was done to provide a cover story for the blue light in the sky.
Donald Fox has studied the JFK assassination since 1992 and 9/11 since 2005. He has done some of the best work on the use of nuclear devices on 9/11. He maintains a blog at http://donaldfox.wordpress.com.
©2013 by Ara Barsamian, NNPI
High Explosive:chemical explosive that has high detonation velocity, usually in excess of 6000 m/s
Hohlraum: black body cavity, same as hydrogen bomb radiation confinement case
Hollow Boosting: refers to fission yield enhancement in a hollow fissile material pit filled with D+T boost gas that releases copious amounts of thermonuclear neutrons
Hydrodynamics: branch of Fluid Dynamics that explores behavior of materials under intense shock pressure; extensively used in implosion calculations to calculate time-dependent compression of pit or canned TN assembly, and thus, supercriticality or thermonuclear burn rate
IHE: Insensitive High Explosive, a very safe type of explosive that can be detonated only by a strong shock; a bullet impact will have no effect
Implosion: uniform squeezing of matter by shock waves or radiation. It can be in 3 dimensions (spherical), 2 dimensions (cylindrical), or 1 dimension (linear)
Implosion-type weapon: assembly of fissile core material by explosive-driven implosion
Initiator: device that provides a squirt of neutrons to start or “initiate” a divergent chain reaction in a super-prompt critical fissile core assembly. There are two basic types: internal Polonium-Beryllium types (original name “Urchin”), and external neutron generator tubes (original name “zipper”
Interstage: usually material containing BeO which absorbs the primary x-rays and re-radiates lower wavelength x-rays over longer time for a more complete secondary assembly implosion and compression
Interval Time: time between the fission primary emission of radiation and the production of thermonuclear output
Isentropic Compression: replaces shock compression with a smooth continuous pressure increase using soft, layered impactors, thereby decreasing heating and allowing much greater compression. Increases yields by a factor of 2 or 3…
Lens: a binary explosive device, where the difference in detonation velocity results in refractive shaping of the output detonation wave; used in early implosion weapons to produce spherical implosions. Superseded by air lenses, flat lenses, and multi-point laser detonation systems.
Levitation: method using a gap between fissile core and tamper, allowing tamper to gain momentum before slamming into the pit; the result is much higher compression due to amplification by the hammer (E=mv2/2) effect
Lithium: metal used either to breed Tritium in reactors to make boost gas, or used in Lithium Deuteride thermonuclear fuel; Li6 isotope has higher cross-section than natural Li7 and is the preferred TN fuel in miniaturized warheads, but both produce Tritium. Ignorance of Li7 cross-section caused the Castle Bravo TN bomb test in 1954 to run away by a factor of 3 yielding 15 MT.
Lithium Deuteride: solid gray salt used as TN fuel in the secondary assembly of a thermonuclear explosive. Usually the Li is enriched to 40 to 60% Li6. Sometime the Li6D is spiked with Tritium ( Sakharov’s 1953 Sloika layer cake design used Li6DT)
Margin: the additional yield of a fission explosive beyond what is needed to drive the explosion of the thermonuclear secondary assembly to full yield
Multipoint Detonation System: replaces the bulky lensed spherical high explosive implosion assembly with multiple individual detonators on the surface of HE sphere. Used successfully in UK in very compact and lightweight Octopus/ super-Octopus system; used in China’s first HEU implosion test (252 detonators). Currently implemented using a high power semiconductor laser and fiber optics cables.
Neutron Initiator: see Initiator
Neptunium: produced in reactors as a by-product, isotope 237 is fissile and can also be used in fission explosives
NTS: Nevada Test Site, used for testing nuclear weapons
Neutron Generator: an integrated assembly of a neutron tube, power supply, timers and triggering system. Some power supplies are electronic, some are explosive ferroelectric or magnetocumulative generators
Neutron Tube: see External Neutron Source
Neutronics: branch of nuclear reactor theory concerned with criticality calculations, such as the variation of degree of criticality with core compression by high explosives
NW: Nuclear weapon
PAL: Permissive Action Link, a mechanical or electronic combination lock preventing the use of a nuclear weapon by unauthorized persons; currently using crypto chips
PBX: Plastic Bonded Explosive, a mix of HE powder pressed together with a plastic binder to make easily handled and machined parts
Pit: the central metal core assembly containing an inner fissile shell of Pu or HEU, usually flashed with gold or Nickel, and an outer shell of either stainless steel, Beryllium, or Vanadium, or a combination; see also Core, and FRP
Pit Tube: a thin stainless steel tube carrying boost gas from the reservoir to the hollow core of the pit at the time of detonation
Plasma: fourth state of matter, whereby the material is completely ionized
Plutonium: element 94. The isotope 239 sustains fast neutron chain reaction and is the fissile material used in fission explosives. Reactors also produce the isotope 240 material, fissionable only by fast neutrons, which has high spontaneous neutron emission that could cause pre-initiation and fizzle yield.
PNE: peaceful nuclear explosives; typically minimizes fission products
Polonium: a high emitter of alpha particles with a half life of 138 days; it was used in combination with Beryllium to generate neutrons for initiating a fission chain reaction
Pre-detonation: detonation of fission explosive before the optimum time, usually before achieving maximum compression of fission core because of high rate of spontaneous fission – see Pre-initiation
Pre-heating: the heating of TN secondary assembly by fission primary before radiation implosion is completed, leading to reduced compression and TN fizzle yield
Pre-initiation: initiation before the optimum time, usually before achieving maximum compression of fission core; causes significant decrease in yield, usually a “fizzle” yield
Primary: primary bomb, usually a fission explosive providing the soft x-ray radiation driving the implosion of a secondary (thermonuclear) bomb to achieve high compression
Radiation Case: metal case containing the primary radiation long enough to implode the secondary; usually stainless steel, aluminum, or plastic with Uranium or Lead coating. Also known as a hohlraum
Radiation Implosion: thermal X-rays from a fission explosive cause ablation of the TN secondary assembly surface, thus driving a strong implosion and high compression of the assembly to make it yield most of the explosive energy. AKA Teller-Ulam principle
Radiation Implosion Compression Mechanism: in the initial US design, the radiation heated a radiation channel filler (foam) to a plasma that pushed against the secondary assembly to compress it (like steam pressure against turbine blades). Radiation driven ablation was discovered accidentally when trying to miniaturize a warhead by using a secondary with a Beryllium-reflected HEU wrap around the LiD fuel and sparkplug; Be is a very good ablator (very high velocity of ablated ejecta) thus providing much greater compression and increased yield from a smaller secondary assembly than “exploding” foam. Latest designs used “doped” or profiled ablators to provide a near isentropic compression, similar to laser fusion capsules. In UK and France, the initial design used the “explosive pusher” concept, where the external metal layer (iron) around the secondary was heated by the thermal x-rays, half blowing outwards, the other half pushing inside, for modest compression.
Reactor-grade Plutonium: Plutonium produced in power reactors with a high content (greater than 20%) of the undesirable isotope 240; unfortunately still a good nuclear explosive material
Reflector: material that reflects neutrons; a good reflector surrounding a fissile core decreases the critical mass of fissile material. Best core reflector is Beryllium metal
Reservoirs: metal containers, usually stainless steel, storing boost gas, deuterium and tritium, under high pressure or absorbed in metals as hydrides
RV: missile re-entry vehicle, housing the nuclear warhead and the arming, fuzing, and firing system, and interface to the missile “bus”
Secondary: secondary or auxiliary bomb, a physically separate component containing thermonuclear fuel such as Lithium Deuteride surrounded by a fusion tamper such as natural Uranium or, for increased yield in a compact package, HEU. Known as Canned Sub-Assembly (CSA)
Separation: process of enriching an element in the percentage of the desired isotope
Shocks: a steep pressure wave, generated by explosives, ballistic impact, electrostatic implosion, etc.
Shot: a nuclear test explosion
SNM: special nuclear material, same as fissile material, Plutonium 239, or HEU
Sparkplug: informal term denoting a fissile core inside a thermonuclear (TN) fuel container that is used to start the thermonuclear burn in the previously compressed TN fuel. Initially used Plutonium, currently HEU
Spontaneous Fission: typically natural spontaneous nuclear fission of Uranium 238 or Plutonium 240 nuclei producing high level of neutron background; main cause of pre-detonation
Sprytron: a gas filled triggered spark-gap tube used as an ultra fast switch to fire the detonators
SRD: Secret Restricted Data, one of the numerous information classification categories
Staging: physical separation of the primary fission stage from the TN secondary stage to allow time for radiation implosion and prevent its destruction by primary material debris
Stockpile: quantity of nuclear weapons ready for use
SSS: Science-based Stockpile Stewardship, a program whereby the stockpile can be certified using computer simulations and statistical sampling and dissection of stockpiled weapons that they are reliable and safe without nuclear testing
Rainbow in the Dark: Powerful proof of 9/11 Nukes
New research falsifies the nanothermite and DEW alternatives while confirming the mini-nuke hypothesis

by Don Fox (with Jim Fetzer)
In accounting for the destruction of the World Trade Center on 9/11, there are three contending factions within the 9/11 research community. While virtually everyone agrees that WTC-7 was brought down in a (classic) controlled demolition, they differ with respect to how the Twin Towers were destroyed, which was obviously not by means of any kind of collapse, which we know, given their design, would have been impossible, but which the government has told the American people was the case. These three factions advance different alternative hypotheses of how it was done:(h1) the nanothermite hypothesis, supported by A&E911, Steve Jones and Christopher Bollyn;
(h2) the mini-nuke hypothesis, supported by Scholars for 9/11 Truth and others here at VT;
(h3) the Directed Energy Weapon (DEW) hypothesis, supported by Judy Wood and her associates.
A&E911 has received enormous support from the public, which has allowed its head, Richard Gage, to travel around the country and lead a comfortable life-style. His critics, however, are skeptical that A&E911 is on the up-and-up because its commitment to nanothernmite has been established to be inadequate as an explanation for the destruction of the Twin Towers, not least of all because in (its extant versions) nanothermite has an explosive force 1/13 as powerful as TNT. While A&E911 apologists maintain the organization does not deny other explosives may have been involved, it has made no effort to ascertain what those “other explosives” could possibly have been, which is inexcusable.
That is rather astonishing, all things considered, because (h1) has been based (virtually exclusively) upon dust samples that were gathered from an apartment in close proximity to “Ground Zero”, where the US Geological Survey has done overwhelmingly more through, detailed and extensive studies of dust samples take from a wide range of locations around Manhattan and subjected them to spectrographic analysis, which has revealed many elements whose presence in the quantities and correlations only appear to be explicable if 9/11 was nuclear event as (h2) asserts:
Barium and Strontium: Neither of these elements should ever appear in building debris in these quantities. The levels never fall below 400ppm for Barium and they never drop below 700ppm for Strontium and reach over 3000ppm for both in the dust sample taken at Broadway and John Streets.These findings expose the pseudo-scientific pretensions of the proponents of (h1) and of (h3) alike, neither of whom take the USGS data into account. That is especially ironic in the case of the “thermite sniffers”, since Steven Jones and A&E911 pride themselves on being “scientific” in their approach, when science requires that reasoning be based upon all the available evidence, yet they ignore more copious and detailed “dust sample” evidence than that upon which they base their own theory. And the “DEW huggers” display their incompetence in precisely the same way by failing to adjust their theory to new evidence and alternative hypotheses. Since I published my review on amazon.com in mid-2012, I have been assailed by an astounding 5,000 attacks by her fans:
Thorium and Uranium: These elements only exist in radioactive form. Thorium is a radioactive element formed from Uranium by decay. It’s very rare and should not be present in building rubble, ever. So once again we have verifiable evidence that a nuclear fission event has taken place.
Lithium: With the presence of lithium we have compelling evidence that this fission pathway of Uranium to Thorium and Helium, with subsequent decay of the Helium into Lithium has taken place.
Lanthanum: Lanthanum is the next element in the disintegration pathway of the element Barium.
Yttrium: The next decay element after Strontium, which further confirms the presence of Barium.
Chromium: The presence of Chromium is one more “tell tale” signature of a nuclear detonation.
Tritium: A very rare element and should not be found at concentrations 55 times normal the basement of WTC-6 no less than 11 days after 9/11, which is another “tell tale” sign of nukes.
Donald Fox has done a brilliant job of exposing the role of Judy Wood as a “gatekeeper extraordinaire”, where the A&E911 (h1) group cannot be far behind. Unfortunately, a gullible public swoons over an organization composed of a thousand or more architects and engineers, even though they will not address WHO was responsible and WHY 9/11 took place and, like Judy Wood and her (h3) group, they do not even provide an adequate explanation for HOW it was done. Here Don Fox advances a new line of argument confirming the use of nukes on 9/11 in support of (h2). This article has a more scientific and technical character than others, where we conclude with a glossary of terms related to the design, construction and detonation of nuclear weapons, the popularization of which Gordon Duff has pioneered, which, alas, must become part of the working knowledge of informed citizens today.
Rainbow in the Dark: Powerful Proof of 9/11 Nukes
by Don Fox
The destruction of the World Trade Center buildings was a high energy event. In the case of the Twin Towers two 110 story buildings were largely converted into millions of cubic yards of very fine dust in about 20 seconds. What was the source of energy that was responsible for the demise of these behemoths? Kerosene has no ability to produce the results observed at Ground Zero on 9/11.
The evidence collected at Ground Zero tells us 9/11 was a nuclear event. The Department of Energy water samples contained tritium which is a rare hydrogen isotope and a telltale sign of a thermonuclear explosion. The USGS dust samples contained barium, strontium, yttrium and cesium amongst other fission products. The earth and water tell us what happened on 9/11: the WTC was nuked.
Most 9/11 researchers (and the general public) lack a basic understanding of nuclear physics. This lack of physics knowledge has allowed people like Steve Jones and Judy Wood to obfuscate the true method of destruction of the WTC buildings with bogus claims of explosive nanothermite and amorphous DEWs. Once one gains an understanding of concepts such as nuclear fission and alpha and beta decay, the destruction of the Twin Towers and the aftermath makes perfect sense.
Nuclear Physics Basics
Atomic Nucleus
There are three parts of an atom: protons, neutron, and electrons. Protons have a positive charge, electrons have a negative charge, and neutrons possess no net charge.
Electrons are the smallest parts of the atom. They are the most numerous of the three. It has no known components or substructure, so it is an elementary particle. Its mass is 1/1836 of a proton. It is also considered to be a fermion. It has an antiparticle called the positron. The positron is identical to the electron except that it carries opposite charge. When an electron collides with a positron, both particles will either scatter or be destroyed producing gamma ray photons. Electrons can collide with other particles and be diffracted like light. Two electrons cannot occupy the same quantum state based on the Pauli Exclusion Principle.
The proton is the part of an atom that helps to form the nucleus and has a positive charge. Protons must have an equal number of neutrons except in the hydrogen atom where a single proton exists on its own. A proton is composed of 2 up quarks and one down quark. They are considered to be fermions and baryons. They are held together by the strong nuclear force. The number of protons in the nucleus of an atom determines the atomic number.
A neutron is the part of an atom that holds no charge. Neutrons and protons occur in equal numbers in stable atoms except in hydrogen. Protons and neutrons are often referred to together as nucleons. If there are more neutrons than protons, then the atom is considered an isotope. If a neutron becomes free of its proton, then it becomes unstable, undergoes beta decay, and will disintegrate in an average of 15 minutes. The neutron is also important in nuclear chain reactions both natural and artificial.
Extensive studies by Charles Boldwyn have demonstrated that the Twin Towers could not have collapsed on 9/11.
From Chemistry Explained.com: The nucleus is composed of protons (charge = +1; mass = 1.007 atomic mass units ([μ]) and neutrons. The number of protons in the nucleus is called the atomic number (Z) and defines which chemical element the nucleus represents. The number of neutrons in the nucleus is called the neutron number (N), whereas the total number of neutrons and protons in the nucleus is referred to as the mass number (A), where A = N + Z. The neutrons and protons are referred to collectively as nucleons. A nucleus with a given N and Z is referred to as a nuclide. Nuclides with the same atomic number are isotopes, such as 12 C and 14 C, whereas nuclides with the same N, such as 14 C and 16 O, are called isotones. Nuclei such as 14 N and 14 C, which have the same mass number, are isobars. Nuclides are designated by a shorthand notation in which one writes, that is, for a nucleus with 6 protons and 8 neutrons, one writes, or just 14 C. The size of a nucleus is approximately 1 to 10 × 10 -15m, with the nuclear radius being represented more precisely as 1.2 × A 1/3 × 10 -15m. We can roughly approximate the nucleus as a sphere and thus we can calculate its density where 1.66 × 10-27 kg is the mass of the nucleon. Thus the nuclear density is about 200,000 tons per cubic millimeter and is independent of A. Imagine a cube that is 1 mm on a side. If filled with nuclear matter, it would have a mass of about 200,000 tons. This calculation demonstrates the enormous matter/energy density of nuclei and gives some idea as to why nuclear phenomena lead to large energy releases.
Of the 6,000 species of nuclei that can exist in the universe, about 2,700 are known, but only 270 of these are stable. The rest are radioactive, that is, they spontaneously decay. The driving force behind all radioactive decay is the ability to produce products of greater stability than one had initially. In other words, radioactive decay releases energy and because of the high energy density of nuclei, that energy release is substantial. Qualitatively we describe radioactive decay as occurring in three general ways: α -, β -, and γ -decay. Alpha-decay occurs in the heavy elements, and consists of the emission of a 4 He nucleus. Beta-decay occurs in nuclei whose N/Z ratio is different from that of a stable nucleus and consists of a transformation of neutrons into protons or vice versa to make the nucleus more stable. Gamma-decay occurs when excited nuclei get rid of some or all of their excitation energy via the emission of electromagnetic radiation, or via the radiationless transfer of energy to orbital electrons.
Nuclear Fission
When uranium-235 undergoes fission, the average of the fragment mass is about 118, but very few fragments near that average are found. It is much more probable to break up into unequal fragments, and the most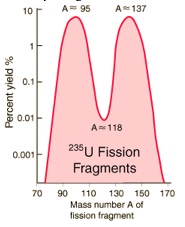 probable
fragment masses are around mass 95 and 137. Most of these fission
fragments are highly unstable (radioactive), and some of them such as cesium-137 and strontium-90 are extremely dangerous when released to the environment.
probable
fragment masses are around mass 95 and 137. Most of these fission
fragments are highly unstable (radioactive), and some of them such as cesium-137 and strontium-90 are extremely dangerous when released to the environment.Cesium-137 and strontium-90 are the most dangerous radioisotopes to the environment in terms of their long-term effects. Their intermediate half-lives of about 30 years suggests that they are not only highly radioactive but that they have a long enough half-life to be around for hundreds of years. Iodine-131 may give a higher initial dose, but its short half-life of 8 days ensures that it will soon be gone. Besides its persistence and high activity, cesium-137 has the further insidious property of being mistaken for potassium by living organisms and taken up as part of the fluid electrolytes. This means that it is passed on up the food chain and re-concentrated from the environment by that process.
Cesium-137 decay has a half-life of 30.07 years and proceeds by both beta decay and gamma emission from an intermediate state. Both the electron and gamma emissions are highly ionizing radiation. The gamma radiation is very penetrating, and the beta radiation, though very short range, is very dangerous when ingested because it deposits all that energy in a very short distance in tissue.
Cesium’s danger as an environmental hazard, damaging when ingested, is made worse by its mimicking of potassium’s chemical properties. This ensures that cesium as a contaminant will be ingested, because potassium is needed by all living things.
Strontium-90 and cesium-137 are the radioisotopes which should be most closely guarded against release into the environment. They both have intermediate half-lives of around 30 years, which is the worst range for half-lives of radioactive contaminants. It ensures that they are not only highly radioactive but also have a long enough half-life to be around for hundreds of years. Strontium-90 mimics the properties of calcium and is taken up by living organisms and made a part of their electrolytes as well as deposited in bones. As a part of the bones, it is not subsequently excreted like cesium-137 would be. It has the potential for causing cancer or damaging the rapidly reproducing bone marrow cells.
Strontium-90 is not quite as likely as cesium-137 to be released as a part of a nuclear reactor accident because it is much less volatile, but is probably the most dangerous component of the radioactive fallout from a nuclear weapon.
Strontium-90 undergoes beta decay, emitting electrons with energy 0.546 MeV with a half-life of 28.8 years. The decay product is yttrium-90.
Beta Decay
Radiation and Radioactive Decay:Beta particles are electrons or positrons (electrons with positive electric charge, or anti-electrons). Beta decay occurs when, in a nucleus with too many protons or too many neutrons, one of the protons or neutrons is transformed into the other. In beta minus decay, a neutron decays into a proton, an electron, and an anti-neutrino: n Æ p + e – +. In beta plus decay, a proton decays into a neutron, a positron, and a neutrino: p Æ n + e+ +n. Both reactions occur because in different regions of the Chart of the Nuclides, one or the other will move the product closer to the region of stability.
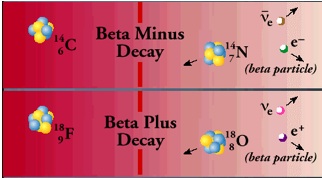
These particular reactions take place because conservation laws are obeyed. Electric charge conservation requires that if an electrically neutral neutron becomes a positively charged proton, an electrically negative particle (in this case, an electron) must also be produced. Similarly, conservation of lepton number requires that if a neutron (lepton number = 0) decays into a proton (lepton number = 0) and an electron (lepton number = 1), a particle with a lepton number of -1 (in this case an antineutrino) must also be produced. The leptons emitted in beta decay did not exist in the nucleus before the decay–they are created at the instant of the decay.
To the best of our knowledge, an isolated proton, a hydrogen nucleus with or without an electron, does not decay. However within a nucleus, the beta decay process can change a proton to a neutron. An isolated neutron is unstable and will decay with a half-life of 10.5 minutes. A neutron in a nucleus will decay if a more stable nucleus results; the half-life of the decay depends on the isotope. If it leads to a more stable nucleus, a proton in a nucleus may capture an electron from the atom (electron capture), and change into a neutron and a neutrino.
Proton decay, neutron decay, and electron capture are three ways in which protons can be changed into neutrons or vice-versa; in each decay there is a change in the atomic number, so that the parent and daughter atoms are different elements. In all three processes, the number A of nucleons remains the same, while both proton number, Z, and neutron number, N, increase or decrease by 1.
In beta decay the change in binding energy appears as the mass energy and kinetic energy of the beta particle, the energy of the neutrino, and the kinetic energy of the recoiling daughter nucleus. The energy of an emitted beta particle from a particular decay can take on a range of values because the energy can be shared in many ways among the three particles while still obeying energy and momentum conservation.
Cherenkov Radiation
Is there an equivalent of the sonic boom for light?When a charged particle does move through a medium at a speed higher than the speed of light in that medium, a faint radiation is produced by the medium. In water, for example, the charged particle excites the water molecules, which then return to their normal state by emitting photons of blue light. Because the particle is moving faster than the speed of light in water, it can trigger a cascade of photons that are in phase with each other and can interfere constructively to form a visible blue glow.
Nuclear Waste Encapsulation and Storage Facility, Cherenkov Radiation, Hanford Site, Southeastern Washington State
Cesium-137 and Strontium-90 Capsules
In the early 1970s operators at the Hanford, Washington, site removed a large fraction of the Cs-137 and Sr-90 from the site’s high-level tank waste in order to reduce the requirements for cooling the tanks. The cesium and strontium were concentrated and sealed in stainless steel (SS) capsules for potential uses, for example, thermoelectric generators and sterilizers. The expected applications for the Hanford capsules did not materialize and ceased entirely in 1988 after one capsule being used in the commercial sector was found to be leaking (USNRC, 1989).

In the capsules, cesium is in the form of cesium chloride (CsCl) and strontium as strontium fluoride (SrF2). The chemical composition has been described as being relatively uniform (NRC, 1997b). Each cesium capsule contains on average approximately 35,000 Ci of Cs-137 plus an unspecified amount of Cs-135 estimated to be 0.7 Ci and produces approximately 190 W of heat. Each strontium capsule contains approximately 33,000 Ci of Sr-90 and produces approximately 260 W of heat.
The blue glow from the cesium-137 and strontium-90 capsules is due to Cherenkov radiation.
Cesium-137 Glows Blue
Majia Nadesan has a blog post that discusses the Goiânia accident where a scavenger punctured a radiotherapy device containing cesium-137 with a screwdriver. A deep blue light came out of the hole.
Cesium and Strontium in the USGS Dust Samples
Both cesium and strontium were found in abundance in the USGS dust samples. Both cesium-137 and strontium-90 undergo β− decay. Cesium-137 decays into barium-137m and strontium-90 decays into yttrium-90. Barium and yttrium were also found in abundance in the USGS dust samples. There can be little doubt that the ominous blue glow in the above picture of Ground Zero is from Cherenkov radiation.
Tribute in Light
The Tribute in Light was first presented on March 11, 2002, six months after 9/11. It is quite stunning:
Beautiful, but misleading. The light memorial was done to provide a cover story for the blue light in the sky.
Conclusion
There can be no doubt that nuclear fission chain reactions took place at Ground Zero on 9/11. Fission products cesium and strontium were found in the USGS dust samples. These radioactive elements underwent beta minus decay and released high energy electrons. This is what caused the blue glow (Cherenkov radiation) over Ground Zero. Jet fuel, nanothermite or a Tesla inspired DEW have no explanatory power for beta minus decay and Cherenkov radiation.Donald Fox has studied the JFK assassination since 1992 and 9/11 since 2005. He has done some of the best work on the use of nuclear devices on 9/11. He maintains a blog at http://donaldfox.wordpress.com.
GLOSSARY OF TERMS:
Nuclear Weapons Glossary©2013 by Ara Barsamian, NNPI
High Explosive:chemical explosive that has high detonation velocity, usually in excess of 6000 m/s
Hohlraum: black body cavity, same as hydrogen bomb radiation confinement case
Hollow Boosting: refers to fission yield enhancement in a hollow fissile material pit filled with D+T boost gas that releases copious amounts of thermonuclear neutrons
Hydrodynamics: branch of Fluid Dynamics that explores behavior of materials under intense shock pressure; extensively used in implosion calculations to calculate time-dependent compression of pit or canned TN assembly, and thus, supercriticality or thermonuclear burn rate
IHE: Insensitive High Explosive, a very safe type of explosive that can be detonated only by a strong shock; a bullet impact will have no effect
Implosion: uniform squeezing of matter by shock waves or radiation. It can be in 3 dimensions (spherical), 2 dimensions (cylindrical), or 1 dimension (linear)
Implosion-type weapon: assembly of fissile core material by explosive-driven implosion
Initiator: device that provides a squirt of neutrons to start or “initiate” a divergent chain reaction in a super-prompt critical fissile core assembly. There are two basic types: internal Polonium-Beryllium types (original name “Urchin”), and external neutron generator tubes (original name “zipper”
Interstage: usually material containing BeO which absorbs the primary x-rays and re-radiates lower wavelength x-rays over longer time for a more complete secondary assembly implosion and compression
Interval Time: time between the fission primary emission of radiation and the production of thermonuclear output
Isentropic Compression: replaces shock compression with a smooth continuous pressure increase using soft, layered impactors, thereby decreasing heating and allowing much greater compression. Increases yields by a factor of 2 or 3…
Lens: a binary explosive device, where the difference in detonation velocity results in refractive shaping of the output detonation wave; used in early implosion weapons to produce spherical implosions. Superseded by air lenses, flat lenses, and multi-point laser detonation systems.
Levitation: method using a gap between fissile core and tamper, allowing tamper to gain momentum before slamming into the pit; the result is much higher compression due to amplification by the hammer (E=mv2/2) effect
Lithium: metal used either to breed Tritium in reactors to make boost gas, or used in Lithium Deuteride thermonuclear fuel; Li6 isotope has higher cross-section than natural Li7 and is the preferred TN fuel in miniaturized warheads, but both produce Tritium. Ignorance of Li7 cross-section caused the Castle Bravo TN bomb test in 1954 to run away by a factor of 3 yielding 15 MT.
Lithium Deuteride: solid gray salt used as TN fuel in the secondary assembly of a thermonuclear explosive. Usually the Li is enriched to 40 to 60% Li6. Sometime the Li6D is spiked with Tritium ( Sakharov’s 1953 Sloika layer cake design used Li6DT)
Margin: the additional yield of a fission explosive beyond what is needed to drive the explosion of the thermonuclear secondary assembly to full yield
Multipoint Detonation System: replaces the bulky lensed spherical high explosive implosion assembly with multiple individual detonators on the surface of HE sphere. Used successfully in UK in very compact and lightweight Octopus/ super-Octopus system; used in China’s first HEU implosion test (252 detonators). Currently implemented using a high power semiconductor laser and fiber optics cables.
Neutron Initiator: see Initiator
Neptunium: produced in reactors as a by-product, isotope 237 is fissile and can also be used in fission explosives
NTS: Nevada Test Site, used for testing nuclear weapons
Neutron Generator: an integrated assembly of a neutron tube, power supply, timers and triggering system. Some power supplies are electronic, some are explosive ferroelectric or magnetocumulative generators
Neutron Tube: see External Neutron Source
Neutronics: branch of nuclear reactor theory concerned with criticality calculations, such as the variation of degree of criticality with core compression by high explosives
NW: Nuclear weapon
PAL: Permissive Action Link, a mechanical or electronic combination lock preventing the use of a nuclear weapon by unauthorized persons; currently using crypto chips
PBX: Plastic Bonded Explosive, a mix of HE powder pressed together with a plastic binder to make easily handled and machined parts
Pit: the central metal core assembly containing an inner fissile shell of Pu or HEU, usually flashed with gold or Nickel, and an outer shell of either stainless steel, Beryllium, or Vanadium, or a combination; see also Core, and FRP
Pit Tube: a thin stainless steel tube carrying boost gas from the reservoir to the hollow core of the pit at the time of detonation
Plasma: fourth state of matter, whereby the material is completely ionized
Plutonium: element 94. The isotope 239 sustains fast neutron chain reaction and is the fissile material used in fission explosives. Reactors also produce the isotope 240 material, fissionable only by fast neutrons, which has high spontaneous neutron emission that could cause pre-initiation and fizzle yield.
PNE: peaceful nuclear explosives; typically minimizes fission products
Polonium: a high emitter of alpha particles with a half life of 138 days; it was used in combination with Beryllium to generate neutrons for initiating a fission chain reaction
Pre-detonation: detonation of fission explosive before the optimum time, usually before achieving maximum compression of fission core because of high rate of spontaneous fission – see Pre-initiation
Pre-heating: the heating of TN secondary assembly by fission primary before radiation implosion is completed, leading to reduced compression and TN fizzle yield
Pre-initiation: initiation before the optimum time, usually before achieving maximum compression of fission core; causes significant decrease in yield, usually a “fizzle” yield
Primary: primary bomb, usually a fission explosive providing the soft x-ray radiation driving the implosion of a secondary (thermonuclear) bomb to achieve high compression
Radiation Case: metal case containing the primary radiation long enough to implode the secondary; usually stainless steel, aluminum, or plastic with Uranium or Lead coating. Also known as a hohlraum
Radiation Implosion: thermal X-rays from a fission explosive cause ablation of the TN secondary assembly surface, thus driving a strong implosion and high compression of the assembly to make it yield most of the explosive energy. AKA Teller-Ulam principle
Radiation Implosion Compression Mechanism: in the initial US design, the radiation heated a radiation channel filler (foam) to a plasma that pushed against the secondary assembly to compress it (like steam pressure against turbine blades). Radiation driven ablation was discovered accidentally when trying to miniaturize a warhead by using a secondary with a Beryllium-reflected HEU wrap around the LiD fuel and sparkplug; Be is a very good ablator (very high velocity of ablated ejecta) thus providing much greater compression and increased yield from a smaller secondary assembly than “exploding” foam. Latest designs used “doped” or profiled ablators to provide a near isentropic compression, similar to laser fusion capsules. In UK and France, the initial design used the “explosive pusher” concept, where the external metal layer (iron) around the secondary was heated by the thermal x-rays, half blowing outwards, the other half pushing inside, for modest compression.
Reactor-grade Plutonium: Plutonium produced in power reactors with a high content (greater than 20%) of the undesirable isotope 240; unfortunately still a good nuclear explosive material
Reflector: material that reflects neutrons; a good reflector surrounding a fissile core decreases the critical mass of fissile material. Best core reflector is Beryllium metal
Reservoirs: metal containers, usually stainless steel, storing boost gas, deuterium and tritium, under high pressure or absorbed in metals as hydrides
RV: missile re-entry vehicle, housing the nuclear warhead and the arming, fuzing, and firing system, and interface to the missile “bus”
Secondary: secondary or auxiliary bomb, a physically separate component containing thermonuclear fuel such as Lithium Deuteride surrounded by a fusion tamper such as natural Uranium or, for increased yield in a compact package, HEU. Known as Canned Sub-Assembly (CSA)
Separation: process of enriching an element in the percentage of the desired isotope
Shocks: a steep pressure wave, generated by explosives, ballistic impact, electrostatic implosion, etc.
Shot: a nuclear test explosion
SNM: special nuclear material, same as fissile material, Plutonium 239, or HEU
Sparkplug: informal term denoting a fissile core inside a thermonuclear (TN) fuel container that is used to start the thermonuclear burn in the previously compressed TN fuel. Initially used Plutonium, currently HEU
Spontaneous Fission: typically natural spontaneous nuclear fission of Uranium 238 or Plutonium 240 nuclei producing high level of neutron background; main cause of pre-detonation
Sprytron: a gas filled triggered spark-gap tube used as an ultra fast switch to fire the detonators
SRD: Secret Restricted Data, one of the numerous information classification categories
Staging: physical separation of the primary fission stage from the TN secondary stage to allow time for radiation implosion and prevent its destruction by primary material debris
Stockpile: quantity of nuclear weapons ready for use
SSS: Science-based Stockpile Stewardship, a program whereby the stockpile can be certified using computer simulations and statistical sampling and dissection of stockpiled weapons that they are reliable and safe without nuclear testing
Jim Fetzer
A
former Marine Corps officer, Jim Fetzer has published widely on the
theoretical foundations of scientific knowledge, computer science,
artificial intelligence, cognitive science, and evolution and mentality.
McKnight Professor Emeritus at the University of Minnesota Duluth, he has also conducted extensive research into the assassination of JFK, the events of 9/11, and the plane crash that killed Sen. Paul Wellstone.
The founder of Scholars for 9/11 Truth, his latest books include The Evolution of Intelligence (2005), The 9/11 Conspiracy (2007), Render Unto Darwin (2007), and The Place of Probability in Science (2010).
McKnight Professor Emeritus at the University of Minnesota Duluth, he has also conducted extensive research into the assassination of JFK, the events of 9/11, and the plane crash that killed Sen. Paul Wellstone.
The founder of Scholars for 9/11 Truth, his latest books include The Evolution of Intelligence (2005), The 9/11 Conspiracy (2007), Render Unto Darwin (2007), and The Place of Probability in Science (2010).
| LOOK ==>> |
Breaking news special: Did Mossad agent Mike Harari brag of organizing 9/11?
on a Special Edition of The Kevin Barrett Show
This show was broadcast February 10, 2011.It is now archived here — Use Player Coming up Thursday at 9:00 am Pacific – 12:00 Noon Eastern – 17:00 GMT
Will be archived here after the broadcast by Thursday evening.
“THE KEVIN BARRETT SHOW”
Breaking news special: Did Mossad agent Mike Harari brag of organizing 9/11? _Dmitri Khalezov Speaks
Email your questions or comments now.
Guest: Dmitri Khalezov, who says that “legendary” Mossad agent Mike Harari admitted to organizing the 9/11 false-flag op.
JB Campbell writes in Veterans Today: “I have been fortunate to be in contact with Dimitri Khalezov, whose blockbuster revelations about 9-11 are about to change our perception of reality for the better just as profoundly as 9-11 changed them for the worse. He has revealed from first-hand knowledge that Mike Harari organized the 9-11 massacre. First-hand means in this case, Mike Harari told him.”
Dmitri Khalezov is the author of The Third Truth About 9/11. Buy here. Download free here.
He offers this brief bio:
Mr. Dimitri A. Khalezov, a former Soviet citizen, a former commissioned officer of the so-called “military unit 46179”, otherwise known as “the Special Control Service” of the 12th Chief Directorate of the Defense Ministry of the USSR. The Special Control Service, also known as the Soviet atomic (later “nuclear”) intelligence was a secret military unit responsible for detecting of nuclear explosions (including underground nuclear tests) of various adversaries of the former USSR as well as responsible for controlling of observance of various international treaties related to nuclear testing and to peaceful nuclear explosions. After September the 11th Khalezov undertook some extensive 9/11 research and proved that the Twin Towers of World Trade Center as well as its building 7 were demolished by three underground thermo-nuclear explosions – which earned the very name “ground zero” to the demolition site. Moreover, he testifies that he knew about the in-built so-called “emergency nuclear demolitions scheme” of the Twin Towers as long ago as back in the ‘80s – while being a serviceman in the Soviet Special Control Service.
Note: I have been skeptical about Mr. Khalezov in the past based on some of his seemingly offbeat or hyperbolic statements such as:
“…almost 99% of so-called ’9/11 truthers’ and full-time 9/11 conspiracy theorists are merely government appointed shills…”
(That estimate sounds a little high…can I really be the only one not getting a government paycheck?!)
But some folks I respect over at Veterans Today take Mr. Khalezov’s testimony very seriously. So I’m going back and looking over his work…and looking forward to our conversation.
Watch Dmitri Khalezov video.
Check out Kevin’s new book: Questioning the War on Terror: A Primer for Obama Voters.
The Kevin Barrett Show is independently produced and hosted by Kevin Barrett and these shows are externally produced content. All externally produced content broadcast on No Lies Radio is the sole responsibility of the program-content producer and is not the responsibility of NoLiesRadio.org. Any questions or concerns should be directed to the content producer.



No comments:
Post a Comment